A new collaborative initiative between UK universities and countries worldwide to share cutting-edge vaccine technology to prevent future global outbreaks of infectious diseases has been awarded £10.5 million from the Department of Health & Social Care (DHSC) and the Engineering and Physical Sciences Research Council (EPSRC). The funding will support the Future Vaccine Manufacturing Research Hub (FVMR Hub), set up originally in 2017 by Imperial College London with University of Bristol as a partner, to continue operations for a further five years, until 2029.
As part of this partnership, world-leading vaccine scientists at Bristol are working with one of Vietnam’s major vaccine manufacturers, Vabiotech, to share their expertise in using a powerful recombinant production technology which relies on a synthetic baculovirus used as a production tool. The technology, pioneered at Bristol, is uniquely suited for producing next-generation vaccines in large quantities in insect cells that can be easily cultured at low cost in Vietnam.
To mark the funding announcement, and to kick-off Vaccine Hub operations, University of Bristol researchers welcomed representatives from the Vietnamese Ministry of Health, the Vietnamese Embassy in the UK, the CEO and research team of Vabiotech, and leading vaccine scientists and Hub partners from Imperial College London with a reception in the Wills Memorial Building. The visit included a tour of Bristol’s high-tech facilities, including robotics laboratories, the cryogenic electron microscopy facility, and the new biosafety level 3 virology laboratories, all of which were established during the COVID-19 pandemic.
Bristol FVMR Hub lead Professor Imre Berger, Director of the Max Planck-Bristol Centre for Minimal Biology and Co-Director of the Bristol BioDesign Institute, said: “Our partner Vabiotech is particularly interested in using the technology we developed in Bristol to produce vaccines to combat rabies and avian flu, both major challenges in Vietnam. We saw just a few years ago how quickly avian flu, which began in Vietnam, developed into a global threat for humans around the world. Together with Vabiotech, we will put cutting-edge technology in place to afford cost-effective protection.
Professor Agnes Nairn, Pro  Vice Chancellor (Global Engagement) at the University of Bristol said: “As a university, we are committed to building research partnerships that help make a positive health impact on populations around the world, of which this initiative is set to do. We are delighted by this new funding for the Vaccine Hub which will see our efforts continue in fighting infectious diseases that have the potential to affect us all.”
Vice Chancellor (Global Engagement) at the University of Bristol said: “As a university, we are committed to building research partnerships that help make a positive health impact on populations around the world, of which this initiative is set to do. We are delighted by this new funding for the Vaccine Hub which will see our efforts continue in fighting infectious diseases that have the potential to affect us all.”
Professor Robin Shattock, Chair in Mucosal Infection and Immunity at Imperial College London and Lead Investigator of the Hub said: “Through the establishment of the Future Vaccine Manufacturing Hub we are looking to exploit the next wave of biotechnology innovation to rapidly respond to emerging outbreaks and empower countries most at risk to infections to meet their local vaccine needs.”
Dr Dat, CEO and Senior Advisor at Vabiotech, said: “Never has it been more important to work together to help prevent and fight the spread of infectious diseases. The new cost-effective vaccine technology, pioneered at Bristol and shared with Vabiotech, is one example which will help many in our country and, ultimately, also others, who will access these vaccines.”
The hubs were announced by Minister for Health and Secondary Care Will Quince. He said: “I’m thrilled that the UK is building on its strong working relationship with global researchers by funding these innovative vaccine hubs, which will support partners across Africa and South East Asia to improve vaccine manufacturing capability.
“These innovative partnerships between British universities and vaccine developers – with £33 million of UK aid funding – will ensure vaccines are accessible to everyone in need, and allow us to future-proof health systems both here and abroad by accelerating the availability of new vaccines for future pandemics.”
The meeting was also attended by University of Bristol’s Professor Jeremy Tavare, Pro-Vice-Chancellor and Executive Dean, Health and Life Sciences; Professor Liang-Fong Wong, Associate Pro Vice-Chancellor for Internationalisation; Professor Michele Barbour, Associate Pro-Vice Chancellor for Enterprise and Innovation; Professor Elek Molnar, International Director of the Faculty of Life Sciences; Professor Nigel Savery, Head of the School of Biochemistry; and Dr Kathleen Sedgley, Mr Wayne Powell and Ms Justyna Gol from the Bristol BioDesign Institute.
The initiative is funded by the Department of Health and Social Care as part of the UK Vaccine Network (UKVN), a UK Aid programme to develop vaccines for diseases with epidemic potential in low and middle-income countries (LMICs).


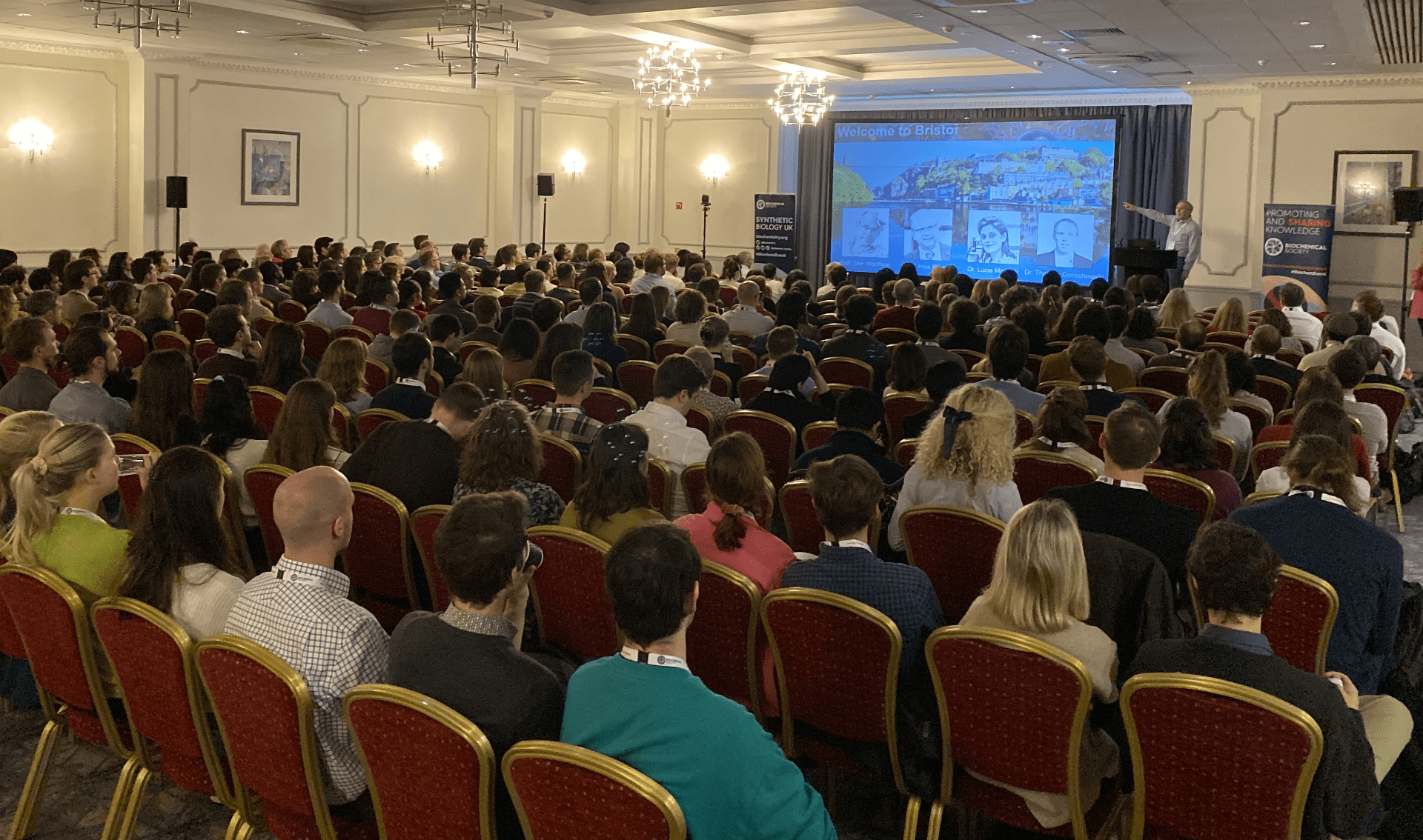 Over 300 people from 45 institutions in 11 countries attended the
Over 300 people from 45 institutions in 11 countries attended the  We would like to thank all the invited speakers (
We would like to thank all the invited speakers ( Congratulations to
Congratulations to  Bringing artificial intelligence (AI) and biosciences together to tackle major societal challenges is the aim of a new five-year £1.6m project involving the University of Bristol and several other UK universities.
Bringing artificial intelligence (AI) and biosciences together to tackle major societal challenges is the aim of a new five-year £1.6m project involving the University of Bristol and several other UK universities.
 Vice Chancellor (Global Engagement) at the University of Bristol said: “As a university, we are committed to building research partnerships that help make a positive health impact on populations around the world, of which this initiative is set to do. We are delighted by this new funding for the Vaccine Hub which will see our efforts continue in fighting infectious diseases that have the potential to affect us all.”
Vice Chancellor (Global Engagement) at the University of Bristol said: “As a university, we are committed to building research partnerships that help make a positive health impact on populations around the world, of which this initiative is set to do. We are delighted by this new funding for the Vaccine Hub which will see our efforts continue in fighting infectious diseases that have the potential to affect us all.”