CEO of Imophoron Ltd, Frederic Garzoni, and University of Bristol researchers, including Imophoron co-founder Professor Imre Berger, combine efforts to generate a promising vaccine candidate for COVID19.

Prior to the pandemic, Frederic Garzoni and Imre Berger bolstered their research in a synthetically engineered protein scaffold named ADDomer™, and with Oracle’s cloud computing technology and Cryo-Electron microscopy (Cryo-EM), the protein was found to be thermotolerant. Professor of Biochemistry, Christiane Berger-Schaffitzel, who was leading the Cryo-EM team, said that “seeing Fred’s vaccine design [at near atomic resolution] in such detail gave us confidence that we were on the right track”.
The protein’s novel ability to maintain its structural integrity without refrigeration, even for months on end, eliminates the cold chain problem that all other mRNA based vaccines are constricted to. To compare, COVID vaccines by AstraZeneca, Moderna and BioNTech need to be cooled at 4°, -20° or even -80°, which restricts their transportability to remote locations.
The protein contains a druggable ‘pocket’ in its surface, which can be injected with various live-attenuated diseases. Professor Imre Berger explains that the body then develops a strong immune response against the “small and harmless pieces of the virus on top of the surface of the ADDomer™”.
First tested on emerging infectious arbovirus, Chikungunya, the vaccine candidate can be used boost antibodies against a host of other viruses and diseases. Spurred on by the success of antibody development in animal models, the Cryo-EM work, accelerated by Oracle Cloud, was instrumental in fast-tracking the vaccine design with other viruses in its ‘pocket’. Today, Imophoron Ltd has secured a £4 million investment to accelerate three vaccines to clinical trials on humans; Chikungunya, RSV (respiratory syncytial virus), and COVID19.
When the first lockdown hit in 2020, a group of clinicians, virologists, chemists, biologists and other academics mobilised as the University COVID19 Emergency Research (UNCOVER) group to tackle the global health crisis. “Scientists everywhere mounted an unprecedented effort to decipher SARS-CoV-2 and the disease, in record time” says Adam Finn, UNCOVER lead and Bristol Professor of Paediatrics. UNCOVER labs urgently needed SARS-CoV-2 antigens to create tests to detect antibodies in the blood of people who had contracted the virus.
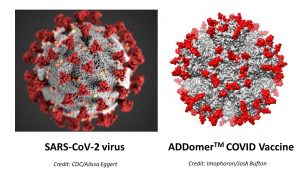
Fred Garzoni volunteered to assist UNCOVER with supplying the reagents they needed, having worked closely with Bristol researchers Berger and Berger-Schaffitzel on ADDomer™. This was also a unique opportunity to test Imophoron’s protein scaffold technology and develop, as fast as possible, a thermotolerant COVID-19 vaccine candidate with the protein. Within weeks, a viable vaccine had been developed for use on animals in preclinical tests.
“We had access to everything and everybody through UNCOVER” said Fred Garzoni, who worked tirelessly with the Vet School and the ASU’s UNCOVER researchers including Mick Bailey, Jamie Mann, Joe Roe, and David Morgan. Their trials on animal models showed incredibly promising results. Not only did the COVID-19 vaccine candidate induce strong immune responses in subjects, but it critically interrupted virus transmissibility.
In addition, the vaccine may not need trained healthcare professionals to administer the dose. The vaccine was just as effective when administered intranasally as with a syringe, which would reduce the cost and complexity of rolling out worldwide pandemic vaccine programmes. In theory, you could self-administer the COVID vaccine as a nasal spray at home!
The distinctive features of this vaccine candidate – its thermostability and various methods it can be administered- truly set the ADDomer™ apart from existing vaccines. Now that the global uptake of the COVID vaccine stands at 47%, this discovery made by Imophoron Ltd and UNCOVER at the University of Bristol could truly transform the accessibility of vaccines to the most remote corners of the world.

